20091028
Wilson et al. BBA-Review mRNA surveillance pathways
Pathways of normal mRNA degradation
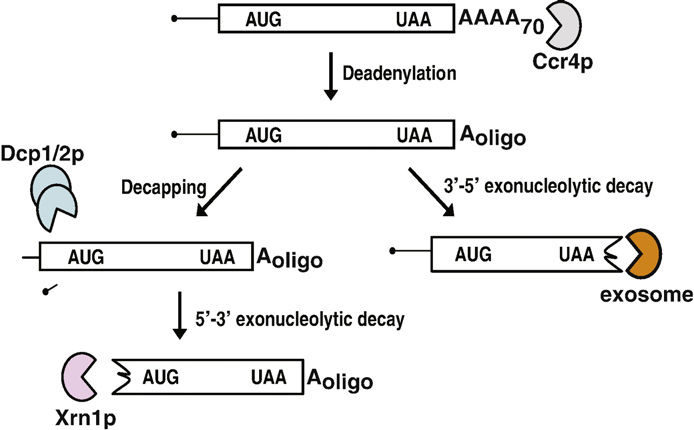
2 major cytoplasmic degradation pathways for normal mRNAs. All of the enzymes required for these two pathways are conserved in other eukaryotes, suggesting that the pathways of mRNA decay are also conserved. Messenger RNA degradation is initiated by gradual removal of the poly(A) tail, a process which is normally carried out by the 3′ exonuclease Ccr4, but also by Pan2, at a slower rate. In the absence of both Ccr4 and Pan2, poly(A) tails are stable, suggesting that there are no other enzymes that can substitute for this function. Removal of the poly(A) tail triggers two mRNA degradation pathways. In the first pathway, the 5′ cap is removed by the Dcp1/Dcp2 complex. Decapping of the mRNa triggers degradation of the transcript from the 5′ end by Xrn1, a 5′ exoribonuclease. In the second pathway, the body of the transcript is degraded from the 3′ end by a multi-subunit 3′ exoribonuclease termed the exosome. The exosome has both nuclear and cytoplasmic functions, both requiring additional factors. For example, Ski2, Ski3, Ski7 and Ski8 are required for all cytoplasmic exosome functions.
NSD-Nonstop mRNA decay
which selectively degrades transcripts that lack all in-frame termination codons. The translation ribosome translates to the end of the poly(A) tail of a nonstop mRNA and stalls. The stalled ribosome is hypothesized to be recognized by Ski7, possibly because of the absence of a codon in the A-site of the ribosome. Recognition by Ski7 recruits the exosome to the nonstop mRNA, resulting in degradation of the transcript.
Several lines of evidence support the current model. First, it is likely that the translating ribosome stalls at the end of a nonstop transcript. Nonstop mRNAs remain physically associated with ribosomes when used in in vitro translation reactions, while ribosomes dissocieate from mRNAs that contain a stop codon. Stalled ribosome-mRNA complexes have been useful tools in studying the sorting of nascent protiens, and are surprisingly stable, as they can be purified by sucrose gradient centrifugation or gel filtration. Thus, unlike DNA or RNA polymerases, ribosomes do not simply dissociate when they reach the end of a temlate, which implies that a specific facgtor (perhaps Ski7) is necessary for disassembly of the ribosome. Similarly, the bacterial ribosome needs trans-acting factors to dissociate when it reaches the end of a nonstop mRNA (i.e. tmRNA and SmpB)
Second, nonstop mRNA decay requires active translation. Nonstop mRNAs are stabilized in wild type yeast cells treated with a translational inhibitor, and in mutant cells depleted of charged tRNAs. More importantly, translation of the nonstop mRNA itself is needed: nonstop mRNA decay is prevented when a stop codon is inserted close to the poly(A) tail, and when a stable structure in the 5’UTR prevents its translation.
Third, a nonstop PGK1pG reporter mRNA was stabilized in yeast strains lacking cytoplasmic exosome function, suggesting that the exosome degrades nonstop mRNA. In contrast, mutations inactivating the decapping enzyme or the 5′ to 3′ exoribonuclease, xrn1p, have large effects on the degradation of normal mRNAs, but do not detectably affect the stability of the nonstop PGK1pG mRNA.
Fourth, based on sequence similarity, Ski7 is a likely candidate for recognizing the stalled ribosome at the end of a nonstop mRNA. The C-terminal domain of Ski7 is homologous to translation factors eEF1A and eRF3. Because eEF1A interacts with the ribosome when the A-site contains a sense codon and eRF3 interacts with the ribosome when the A-site contains a stop codon, it is proposed that the homologous domain of Ski7 interacts with the ribosome when the A-site is empty. This hypothesis is supported by the observation that deletion of the C-terminal domain of Ski7 inactivates the nonstop mRNA decay pathway without affecting other exosome functions.
Fifth, the N-terminal domain of Ski7 inteacts with the exosome, and with additional exosome cofactors. This domain is required for both nonstop mRNA deacay and other cytoplasmic exosome functions. Importantly, a point mutation in the exosome that disrupts its interaction with Ski7 also blocks nonstop mRNA decay, confirming that the interaction between Ski7 and the exosome is functioally important.
The exosome degrades the poly(A) tail on nonstop mRNAs is surprising since the exosome is incapable of degrading the poly(A) tail on normal mRNAs, as shown by a complete absence of deadenylation in a ccr4Δpan2Δ double mutant. The dependence on translation and the independence of a distinct deadenylation phase appear to be conserved aspects of nonstop mRNA decay.
2 questions à Résoudre:
1. Whether the translation rate of a nonstop mRNA is the same as for a control mRNA?
2. The fate of the protein produced from a nonstop mRNA is also unclear.
Reports from 2 groups indicate that nonstop proteins may be targeted for proteolysis by the proteasome. Inhibitors of the proteasome increased the levels of the protein encoded by nonstop mRNAs, and a screen to identify trans-acting factors in nonstop mRNA decay uncovered three mutations that inactivate the proteasome. Interestingly, the effects of the proteasome and Ski7 are additive, suggesting that recognition of the stalled ribosome by Ski7 is not required for the rapid degradation of the encoded protein.
Premature polyadenylation generates nonstop mRNAs
1.2% of random yeast cDNA clones and 0.7% of human cDNAs were prematurely polyadenylated. Premature polyadenylation can also serve as a method of gene-specific regulation. In S. cerevisiae, 0.8% of all open reading frames contain a cryptic plyadenylation site upstream of the normal termination codon. Cryptic sites could be favored over the normal poly(A) site under various conditions to down-regulate the expression of the normal mRNA.This implies that premature polyadenylation, coupled with nonstop mRNA decay, could serve to down-regulated many genes.
NGD-No Go mRNA decay
Pseudoknots, rare codons, or stem-loops within an open reading frame cause the translating ribosome to stall. Transcripts with stalled ribosomes are degraded by the no-go mRNA surveillance pathways. A stalled ribosome within the coding region of the no-go transcript is thought to recruit Dom34 and Hbs1. Dom34 and Hbs1 are homologous to eRF1 and eRF3, respectively, suggesting that they may function to recognize stalled ribosome. Interaction of Dom34 and Hbs1 with the stalled riboosme is thought to trigger endonculeolytic cleavage of the no-go mRNA. The resulting 5″ cleavage product is degraded by the cytoplasmic exosome, while the 3′ cleavage product is degraded by the 5′ exoribonuclease, Xnr1. Consistent with this model, an xrn1Δ strain accumulates the expected 3′ cleavage product, but deleting DOM34 from this strain prevents this accumulation. Likewise, a strin lacking cytoplasmic exosome activity accumulates the expected 5′ cleavage product, and deletion DOM34 from this strin prevents this accumulation. Similarly, mutants in HBS1 show drastically reduced levels of no-go mRNA decay intermediates, but hbs1Δ is not as effective as dom34Δ, implying that Hbs1 may not play as great a role as Dom34 in this pathway. Consistent with this observation, recent structrural work suggests that Dom34 is responsbile for endonucleolytic cleavage of no-go mRNA.
Ribosomes can also stalled because of aberrancies within the ribosome. Mutations that are prefentially degraded through the nonfunctional ribosomes that are preferentially degraded through the nonfunctional ribosome decay pathway (NRD). Thus, in the cas of no-go decay, a stalled ribosome leads to mRNA decay, while in NRD, a stalled ribosome leads to rRNA decay. Yet in other cases of ribosomal stalling, yeast mRNAs are stabilized ( e.g. treatment with cycloheximide or depletion of charged tRNA in a cca1-1 stain). Thus, an important question yet to be addressed is how the cellular machinery distinguishes between whether the mRNA or rRNA in a stalled complex is defective, or whether in both cases both mRNA and rRNA are degraded.
An interesting aspect of the relationship between nonstop and no-go decay is that Ski7 and Hbs1 are a pair of duplicated genes in yeast, whereas most other eukaryotes have only one homolog. The single Ski7/Hbs1 homolog from the related yeast Saccharomyces kluyveri could complement the growth phenotypes of an HBS1 mutant and a SKI7 mutant in S. cerevisiae. In contrast, the human homolog of Hbs1/Ski7 is unable to complement either an HBS1 mutant or a SKI7 mutant in S. c. the most likely explanation is that human eRFS is nonfunctional when expressed in yeast, leaving the question whether eRFS can function in nonstop and/or no-go decay unanswered. The evolutionary history of Hbs1p and Ski7 suggests the possibility taht in most eukaryotes, the single Ski7/Hbs1 protein recognizes stalled ribosomes within the coding region and at the end of an mRNA. This also suggests taht in other eukaryotes, nonstop and no-go mRNA decay may be a mixture of endonucleolytic decay and exosome-mediated decay.
20091025
Fernandez et al. 2004 MBC-(Lsm2-Lsm7 complex)
In yeast, Lsm2-Lsm8 complex binds and stabilizes the 3′ end of the spliceosomal U6 snRNA, the Lsm2-8 proteins likely form a heteroheptameric ring;
whereas the Lsm1-Lsm7 complex functions in mRNA decay, Capped mRNA degradation intermediates accumulate in yeast containing mutations in these proteins, indicating that Lsm1-7 complex functions in mRNA decapping. As 3′-shortened mRNAs accumulate in mutant strains, the Lsm1-7 complex may protects 3′ ends of deadelylated mRNAs from nculeases. Immunofluorescence experiments reveal that Lsm1 is mostly cytoplasmic, whereas Lsm7, a component of both complexes, is nuclear and cytoplasmic. Within the cytoplasm, Lsm1 localizes to structures called p-bodies, whch represent sites of mRNA degradation. Thus, the Lsm2-8 complex may be nuclear, consistent with its role as a U6 snRNP component, whereas the Lsm1-7 complex appears largely cytoplasmic.
Lsm2-Lsm7 associates with snR5, a box H/ACA snoRNA that functions to guide site-specific pseudouridylation of rRNA. snR5, this RNA is a member of the box H/ACA class of snoRNAs that fucntion in pseudouridylation of rRNA. Thre are >20 box H/ACA snoRNAs in yeast, all of which are bound by four core proteins: Gar1, Nhp2, Nop10, and the pseudouridine synthase Cbf5. Approximately half the snR5 RNA in cells i sbound by Lsm proteins. The Lsm complex bound to snR5 is distinct from Lsm2-8 and Lsm1-7 complexes, because neither Lsm1 nor Lsm8 are associated with the snoRNA. Experiments in which binding of Lsm proteins to snR5 was reconstituted in vitro reveal that the 3′ end of snR5 is required for Lsm protein recognition. We demonstrate that components of the Lsm2-7 complex are prsent in nucleoli. Interestingly, biochemical fractionation and immunoprecipitation experiments suggest that at least some of the Lsm2-7/snR5 complex is distinct from the fraction of the snR5 bound by Gar1 and Nhp2. Consistent with a separate complex, Lsm proteins are not required for the function of snR5 in pseudouridylation.
Spiller et al. 2007 Jounal of cell science-(Lsm2-8 complex)
Nuclear accumulation of Lsm8 requires Kap95. Production of recombinant human LSM proteins in bacteria, followed by injection of thse prteins into HeLa cells, showed that the pre-assembled LSM2-8 complex localized to the nculeus, whereas LSM injected by itself accumulated in the cytoplasm. These results suggest that LSM8 nuclear import involves an unidentified nuclear-import signal that is only present when LSM8 interacts with other LSM2-8 subunits.
The three paralogues yeast Lsm proteins, Lsm8, Lsm2, Lsm4 also contain basic C-termini, they might form a nculear-localization signal in a similar fashion. Mutations in yeast Lsm8 are suppressed by overexpression of LSM2 or LSM4.
Under normal physiological conditions, competition between Lsm1 and Lsm8 might provide a link between RNA processing events in the nucleus and mRNA degradation in the cytoplasm.
Lsm proteins are actively imported through the nuclear pore
nup49-313 (a nuclear-pore mutant taht affects protein import), strain ts
In contrast to the effect of the nup49-313 mutation on Lsm protein localization, the xpo1-1 nuclear-export mutation showed no effect on the localization of Lsm1, Lsm7 or Lsm8. Thus, it seems that the cytoplasmic localization of Lsm1 is not a consequence of nuclear exclusion by continual active export from the nculeus, at least not through this export pathway.
Nuclear localization of Lsm7 requires other Lsm proteins (not Lsm1)
lsm1Δ and lsm6Δ heat sensitive
Depletion of Lsm2 or Lsm4 disrupts Lsm8 localization
Lsm8 by itself does not accumulate in the nucleus, because loss of either of its proposed partners in the lsm2-8 ring results in its delocalization. However, it seems that a complete Lsm2-8 complex is not essential, because lack of Lsm6 has no effect, which might be expected because Lsm6 is a non-essential protein.
Lsm7 and Lsm8 delocalization is not caused by defective splicing
depletion of Lsm2-8 proteins leads to decreased levels of U6 snRNA and an accumulation of per-mRNA.
Lsm8 truncations affect its nuclear localization, Lsm8 needs to interact with other Lsm proteins for its nculear accumulation
Over-production of Lsm1 or Lsm8 has opposing effects on Lsm7 localization
lsm8-1 mutation is synthetic lethal with deletion of LHP1 (which encodes the yeast homolog of La, another U6 RNA-binding protein), and that the requirement for Lhp1 in an lsm8-1 strain can be suppressed by low-copy overexpressiono f LSM2.
The proposed existence of an Lsm2-7 complex that associates with snR5 in thenucleolus is in apparecontradiction with our finding of Lsm8 requirement for nuclear localization. It seems possible that the full Lsm2-8 complex might interact with snR5, but that the Lsm8 epitope tag might be masked in the snR5 RNP.
Pannone et al. 2001 Genetics-(lsm2-8 complex)
A model of Lsm2 and Lsm4 contact Lsm8 in the Lsm2-8 ring.
Deletion of LSM5, LSM6, or LSM7, but not LSM1, are synthetically lethal with deletions of LHP1. Lhp1 acts redundantly with the assembled Lsm2-8 complex to stabilize newly synthesized U6 RNA.
-
Récent
-
Liens
-
Archives
- Mai 2012 (2)
- décembre 2009 (1)
- octobre 2009 (2)
- septembre 2009 (1)
- juillet 2009 (2)
- avril 2009 (4)
- mars 2009 (6)
-
Catégories
-
RSS
Entries RSS
Comments RSS